Welcome to the ultimate resource for mastering oxidation numbers! Our comprehensive oxidation number worksheet and answers provide an engaging and interactive learning experience that will equip you with a deep understanding of this fundamental chemical concept. Get ready to unravel the mysteries of oxidation numbers and unlock your potential in chemistry.
Throughout this guide, we will explore the intricacies of oxidation numbers, from their definitions and rules to their practical applications. Our detailed explanations, clear examples, and thought-provoking exercises will guide you every step of the way, ensuring a thorough grasp of this essential topic.
Oxidation Number Definitions: Oxidation Number Worksheet And Answers

Oxidation numbers are numerical values assigned to atoms in a chemical compound to represent their relative oxidation state. They reflect the number of electrons that an atom has gained or lost in forming chemical bonds.
For example, in sodium chloride (NaCl), sodium has an oxidation number of +1, while chlorine has an oxidation number of -1. This indicates that sodium has lost one electron to chlorine, resulting in the formation of the ionic bond.
Oxidation Number Rules
- The oxidation number of an element in its elemental form is 0.
- The oxidation number of a monatomic ion is equal to its charge.
- The sum of the oxidation numbers of all atoms in a neutral compound is 0.
- The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
- Hydrogen has an oxidation number of +1 when bonded to nonmetals and -1 when bonded to metals.
- Oxygen has an oxidation number of -2 in most compounds.
Exceptions to the Rules:
- Hydrogen has an oxidation number of -1 in metal hydrides.
- Oxygen has an oxidation number of -1 in peroxides.
- Fluorine always has an oxidation number of -1.
Worksheet Analysis
A common mistake made by students is assigning an incorrect oxidation number to an atom based on its electronegativity. It’s important to remember that oxidation numbers are not based on electronegativity but rather on the number of electrons gained or lost.
Another common mistake is forgetting to consider the charge of the ion when assigning oxidation numbers. For example, in the compound potassium permanganate (KMnO 4), manganese has an oxidation number of +7, not +5, because the permanganate ion has a charge of -1.
Answer Key Examination
The answer key for the oxidation number worksheet should provide clear and concise explanations for each answer. It should also include references to the oxidation number rules and exceptions to support the answers.
One area where the answer key could be improved is by providing more detailed explanations for complex or ambiguous cases. For example, in the case of transition metals, which can have variable oxidation numbers, the answer key should explain the factors that determine the specific oxidation number in each case.
Table of Oxidation Numbers
| Element Symbol | Element Name | Common Oxidation Numbers |
|---|---|---|
| H | Hydrogen | +1,
|
| O | Oxygen | -2,
|
| Na | Sodium | +1 |
| Cl | Chlorine | -1 |
| Fe | Iron | +2, +3 |
Oxidation Number Applications
Oxidation numbers have practical applications in various fields of chemistry, including:
- Balancing chemical equations:Oxidation numbers help identify the changes in oxidation states of atoms during a chemical reaction, making it easier to balance the equation.
- Predicting chemical reactions:By comparing the oxidation numbers of reactants and products, it’s possible to predict the feasibility and direction of a chemical reaction.
Case Studies and Examples
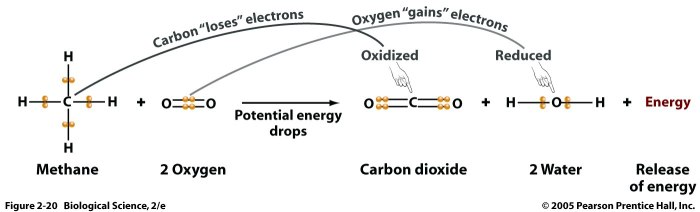
In electrochemistry, oxidation numbers are used to determine the anode and cathode reactions in a voltaic cell. The anode is where oxidation occurs, resulting in an increase in oxidation number, while the cathode is where reduction occurs, resulting in a decrease in oxidation number.
In biochemistry, oxidation numbers are used to understand the mechanisms of enzymatic reactions. Enzymes often catalyze redox reactions, where electrons are transferred between molecules, and oxidation numbers help track these electron transfers.
Teaching Strategies, Oxidation number worksheet and answers
To effectively teach oxidation numbers, it’s important to:
- Start with a clear explanation of the concept and its importance.
- Provide ample practice opportunities through worksheets and exercises.
- Use visual aids, such as diagrams and animations, to illustrate the concept.
- Incorporate hands-on activities, such as experiments or simulations, to enhance understanding.
Common Queries
What are oxidation numbers?
Oxidation numbers represent the hypothetical charge assigned to an atom in a molecule or ion, based on the assumption that electrons are transferred completely from one atom to another.
How do I determine the oxidation number of an element?
Follow the general rules for assigning oxidation numbers, considering the element’s position in the periodic table, its bonding with other atoms, and any charges present.
Why are oxidation numbers important?
Oxidation numbers play a crucial role in balancing chemical equations, predicting the products of reactions, and understanding the electronic structure of molecules.