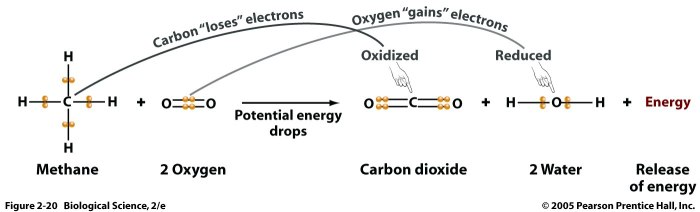
Example of redox and metathesis reactions offer a captivating glimpse into the intricate world of chemical transformations. These reactions play a pivotal role in numerous natural processes and industrial applications, making them indispensable in various scientific disciplines.
Redox reactions involve the transfer of electrons between atoms or ions, leading to changes in their oxidation states. Metathesis reactions, on the other hand, focus on the exchange of ions between compounds, resulting in the formation of new substances with altered compositions.
1. Redox Reactions

Redox reactions involve the transfer of electrons between atoms or ions, resulting in changes in their oxidation states. They are characterized by the simultaneous occurrence of oxidation (loss of electrons) and reduction (gain of electrons).
Examples of Redox Reactions
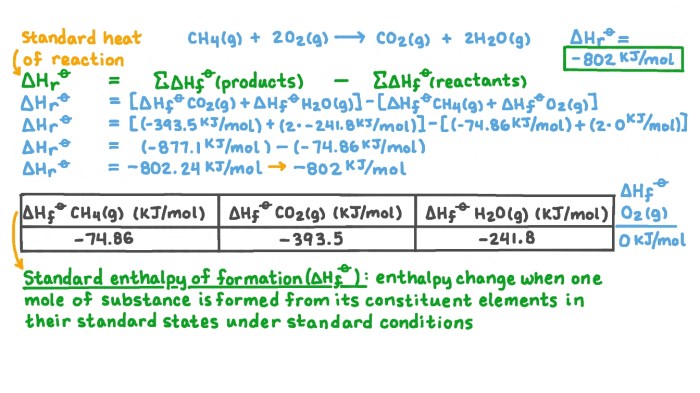
- Combustion of methane: CH 4+ 2O 2→ CO 2+ 2H 2O
- Rusting of iron: 4Fe + 3O 2→ 2Fe 2O 3
- Electrolysis of water: 2H 2O → 2H 2+ O 2
Role of Oxidizing and Reducing Agents
Oxidizing agents are substances that cause oxidation, while reducing agents cause reduction. In a redox reaction, the oxidizing agent is reduced, and the reducing agent is oxidized.
2. Metathesis Reactions
Metathesis reactions are chemical reactions in which two compounds exchange ions to form two new compounds. They are characterized by the formation of a precipitate or a gas.
Examples of Metathesis Reactions
- Precipitation of silver chloride: AgNO 3+ NaCl → AgCl + NaNO 3
- Formation of carbon dioxide: CaCO 3+ 2HCl → CaCl 2+ CO 2+ H 2O
- Double displacement reaction: CuSO 4+ Ba(OH) 2→ Cu(OH) 2+ BaSO 4
Conditions and Mechanisms
Metathesis reactions typically occur in aqueous solutions and involve the exchange of cations or anions between two ionic compounds. The reaction proceeds to form a precipitate or a gas, which drives the reaction to completion.
3. Comparison of Redox and Metathesis Reactions: Example Of Redox And Metathesis Reactions
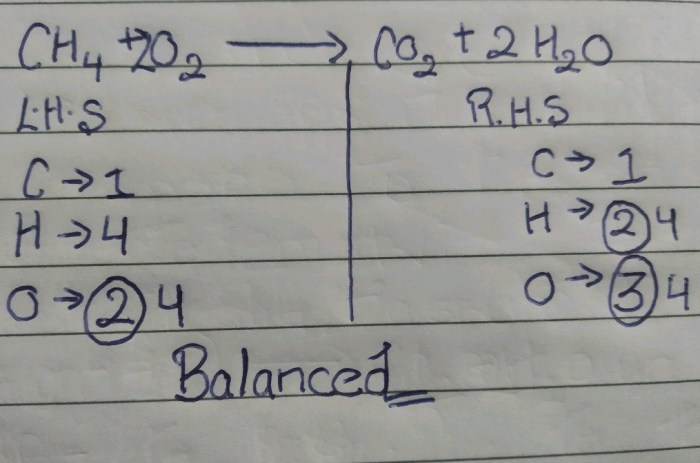
Mechanisms
Redox reactions involve electron transfer, while metathesis reactions involve ion exchange.
Similarities and Differences
- Similarities:Both types of reactions involve the formation of new substances.
- Differences:Redox reactions involve changes in oxidation states, while metathesis reactions do not.
Applications, Example of redox and metathesis reactions
- Redox reactions:Batteries, fuel cells, corrosion prevention
- Metathesis reactions:Preparation of precipitates, gas production, synthesis of new materials
Helpful Answers
What is the difference between a redox reaction and a metathesis reaction?
Redox reactions involve electron transfer, while metathesis reactions involve ion exchange.
What are some examples of redox reactions?
Rusting of iron, combustion of fuels, and photosynthesis are all examples of redox reactions.
What are some examples of metathesis reactions?
The reaction between sodium chloride and silver nitrate to form silver chloride and sodium nitrate is an example of a metathesis reaction.