Embark on a scientific journey with our dehydration synthesis and hydrolysis worksheet, meticulously crafted to provide an in-depth understanding of these fundamental chemical reactions. Through engaging activities and thought-provoking exercises, you will unravel the intricacies of these processes, their significance in biological systems, and their wide-ranging applications.
Delve into the mechanisms driving dehydration synthesis and hydrolysis, exploring the step-by-step transformations that shape these reactions. Discover the factors that govern their rates and delve into the practical applications that harness their power in diverse fields.
Introduction
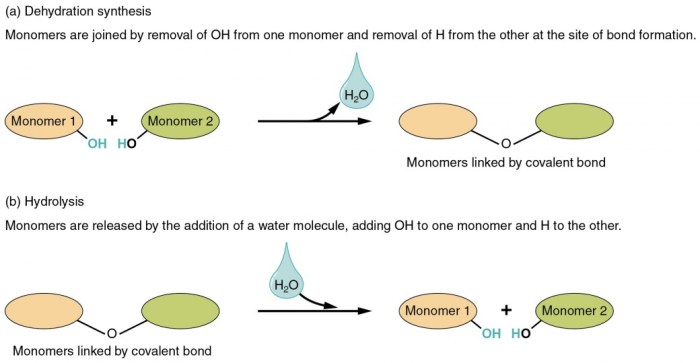
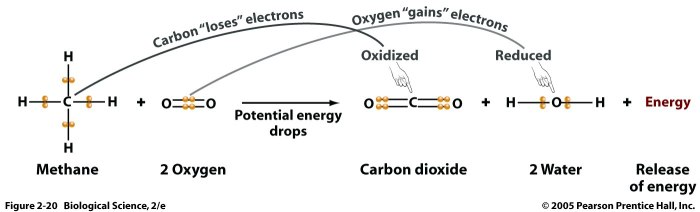
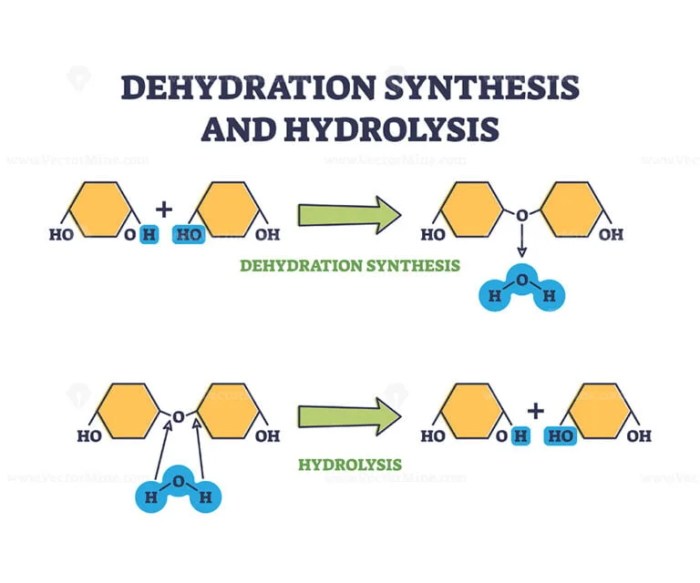
Dehydration synthesis and hydrolysis reactions are fundamental chemical processes that play crucial roles in biological systems. Dehydration synthesis involves the removal of a water molecule to form a covalent bond between two molecules, while hydrolysis involves the addition of a water molecule to break a covalent bond.
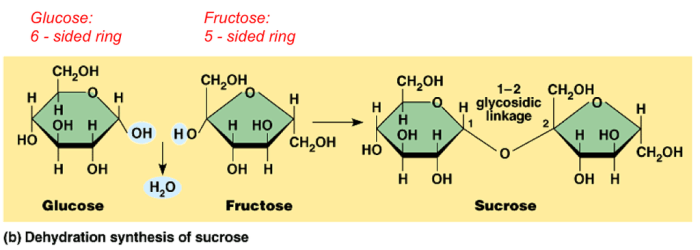
Examples of dehydration synthesis include the formation of peptides from amino acids and the synthesis of polysaccharides from monosaccharides. Hydrolysis reactions, on the other hand, are involved in the breakdown of proteins, carbohydrates, and lipids during digestion.
Mechanisms of Dehydration Synthesis and Hydrolysis
Dehydration Synthesis, Dehydration synthesis and hydrolysis worksheet
Dehydration synthesis reactions typically involve three steps:
- Protonation of the nucleophile
- Nucleophilic attack on the electrophile
- Elimination of a water molecule
Hydrolysis
Hydrolysis reactions, on the other hand, involve the reverse process:
- Protonation of the electrophile
- Nucleophilic attack by water
- Elimination of a leaving group
Factors Affecting Dehydration Synthesis and Hydrolysis: Dehydration Synthesis And Hydrolysis Worksheet
Dehydration Synthesis, Dehydration synthesis and hydrolysis worksheet
The rate of dehydration synthesis reactions is affected by several factors, including:
- Concentration of reactants
- Temperature
- pH
- Presence of a catalyst
Hydrolysis
Similarly, the rate of hydrolysis reactions is affected by:
- Concentration of reactants
- Temperature
- pH
- Nature of the leaving group
Applications of Dehydration Synthesis and Hydrolysis
Dehydration Synthesis, Dehydration synthesis and hydrolysis worksheet
Dehydration synthesis reactions are widely used in industry and research:
- Production of polymers, such as polyethylene and nylon
- Synthesis of pharmaceuticals and other organic compounds
- Condensation reactions in DNA and RNA
Hydrolysis
Hydrolysis reactions are also essential in various applications:
- Digestion of food
- Breakdown of biological molecules in cells
- Industrial processes, such as the hydrolysis of cellulose to produce biofuels
FAQ Overview
What is the difference between dehydration synthesis and hydrolysis?
Dehydration synthesis involves the removal of water to form a larger molecule, while hydrolysis involves the addition of water to break down a larger molecule.
Why are dehydration synthesis and hydrolysis important in biological systems?
These reactions are crucial for building and breaking down complex molecules, such as proteins, carbohydrates, and lipids, which are essential for cellular function.
How can the rates of dehydration synthesis and hydrolysis be controlled?
Factors such as temperature, pH, and the presence of catalysts can influence the rates of these reactions.