Delve into the enigmatic world of chemical reactions with our comprehensive Chemical Reaction Webquest Answer Key, meticulously crafted to illuminate the fundamental principles and applications of this captivating scientific phenomenon. This guide serves as an invaluable resource for students, educators, and anyone eager to unravel the secrets of chemical transformations.
Chemical Reactions: Chemical Reaction Webquest Answer Key
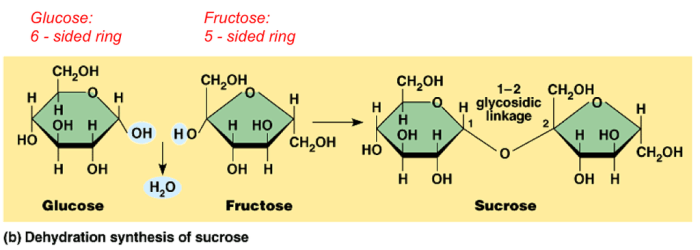
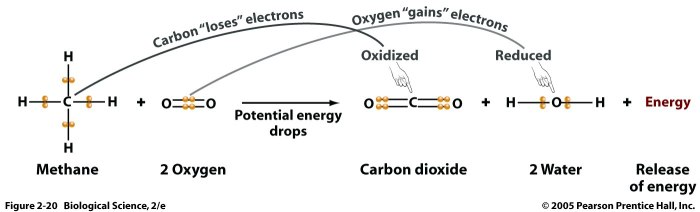
A chemical reaction is a process in which one or more substances, the reactants, are transformed into one or more different substances, the products.
Chemical reactions are represented by chemical equations, which show the chemical formulas of the reactants and products, as well as the stoichiometry of the reaction. The stoichiometry of a reaction is the relative number of moles of each reactant and product involved in the reaction.
Types of Chemical Reactions
There are many different types of chemical reactions, each with its own characteristics. Some of the most common types of reactions include:
- Combination reactionsoccur when two or more substances combine to form a single product.
- Decomposition reactionsoccur when a single substance breaks down into two or more products.
- Single-displacement reactionsoccur when one element replaces another element in a compound.
- Double-displacement reactionsoccur when two compounds exchange ions to form two new compounds.
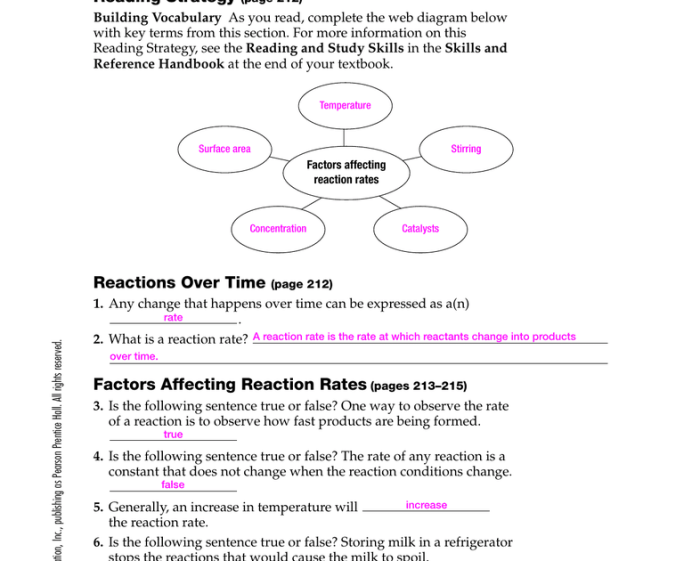
Factors Affecting Chemical Reactions
The rate of a chemical reaction is affected by a number of factors, including:
- Concentration of the reactants: The higher the concentration of the reactants, the faster the reaction will occur.
- Temperature: The higher the temperature, the faster the reaction will occur.
- Surface area of the reactants: The greater the surface area of the reactants, the faster the reaction will occur.
- Presence of a catalyst: A catalyst is a substance that speeds up a chemical reaction without being consumed in the reaction.
Applications of Chemical Reactions, Chemical reaction webquest answer key
Chemical reactions are used in a wide variety of applications, including:
- Energy production: Chemical reactions are used to produce energy in a variety of ways, including burning fossil fuels, nuclear reactions, and solar energy.
- Manufacturing: Chemical reactions are used to manufacture a wide variety of products, including plastics, fertilizers, and pharmaceuticals.
- Medicine: Chemical reactions are used to develop new drugs and treatments for diseases.
Safety Considerations
Chemical reactions can be hazardous, so it is important to take safety precautions when conducting chemical experiments. Some of the most important safety precautions include:
- Wear appropriate safety gear, including gloves, goggles, and a lab coat.
- Work in a well-ventilated area.
- Never mix chemicals unless you know what the reaction will be.
- Dispose of chemicals properly.
Detailed FAQs
What is the definition of a chemical reaction?
A chemical reaction is a process that involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties.
How can I identify different types of chemical reactions?
Chemical reactions can be classified based on various criteria, such as the type of change that occurs (e.g., combination, decomposition, single displacement) or the energy involved (e.g., exothermic, endothermic).
What factors influence the rate of a chemical reaction?
The rate of a chemical reaction can be affected by factors such as temperature, concentration of reactants, presence of a catalyst, and surface area.